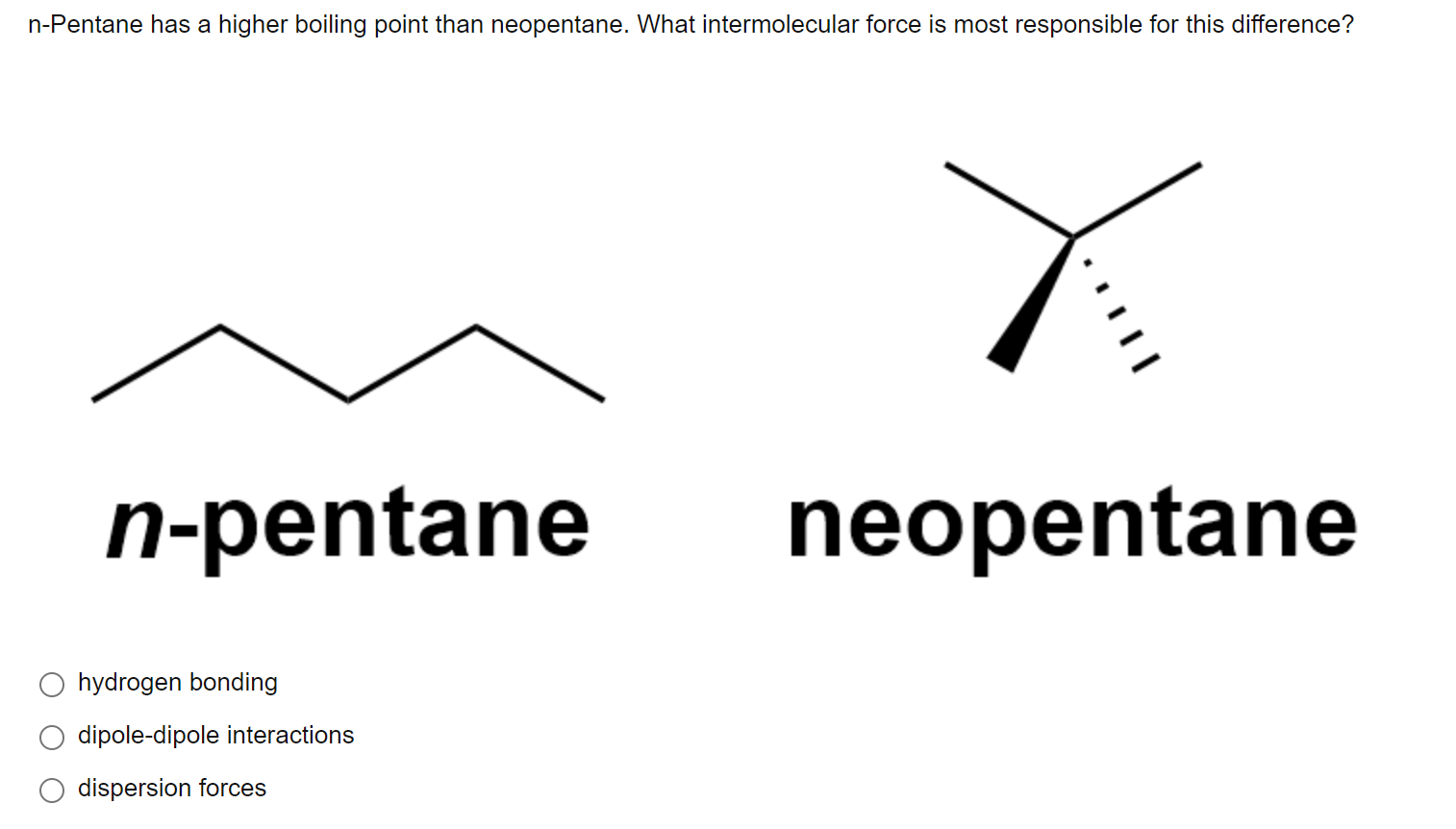
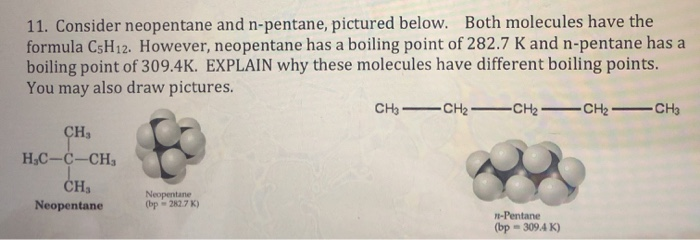
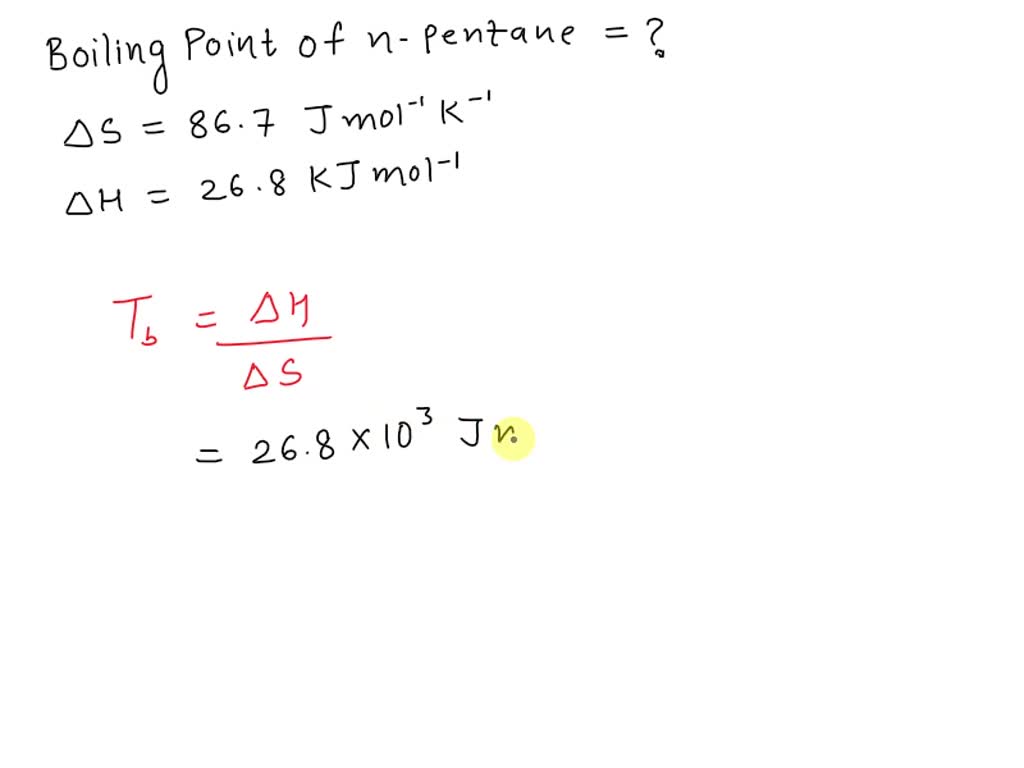Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane

Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane
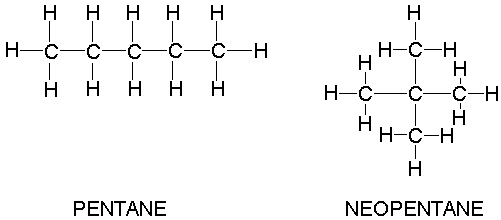
Why when the shape of molecules become more compact it's boiling point decrease while when intermolecular force become strong boiling point increase? | Socratic

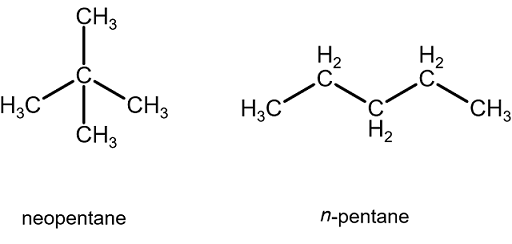
why neopentane has higher melting point than n pentane - Chemistry - Chemical Bonding and Molecular Structure - 13416933 | Meritnation.com
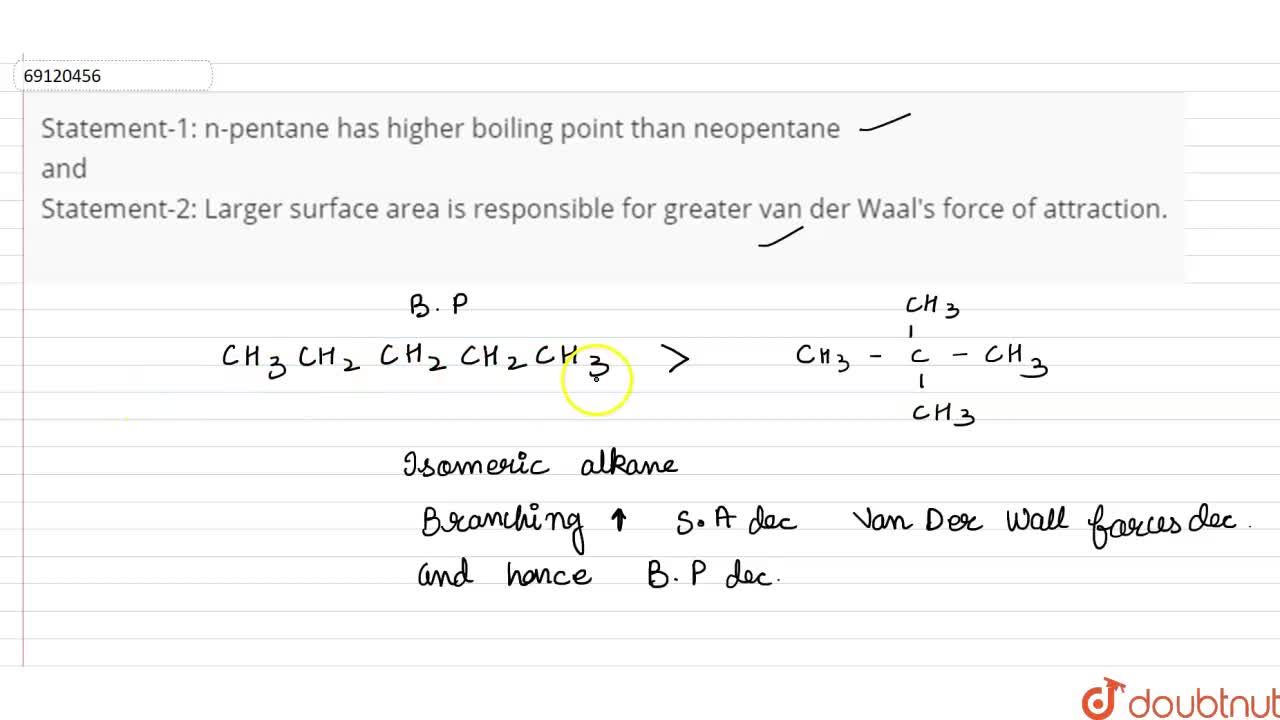
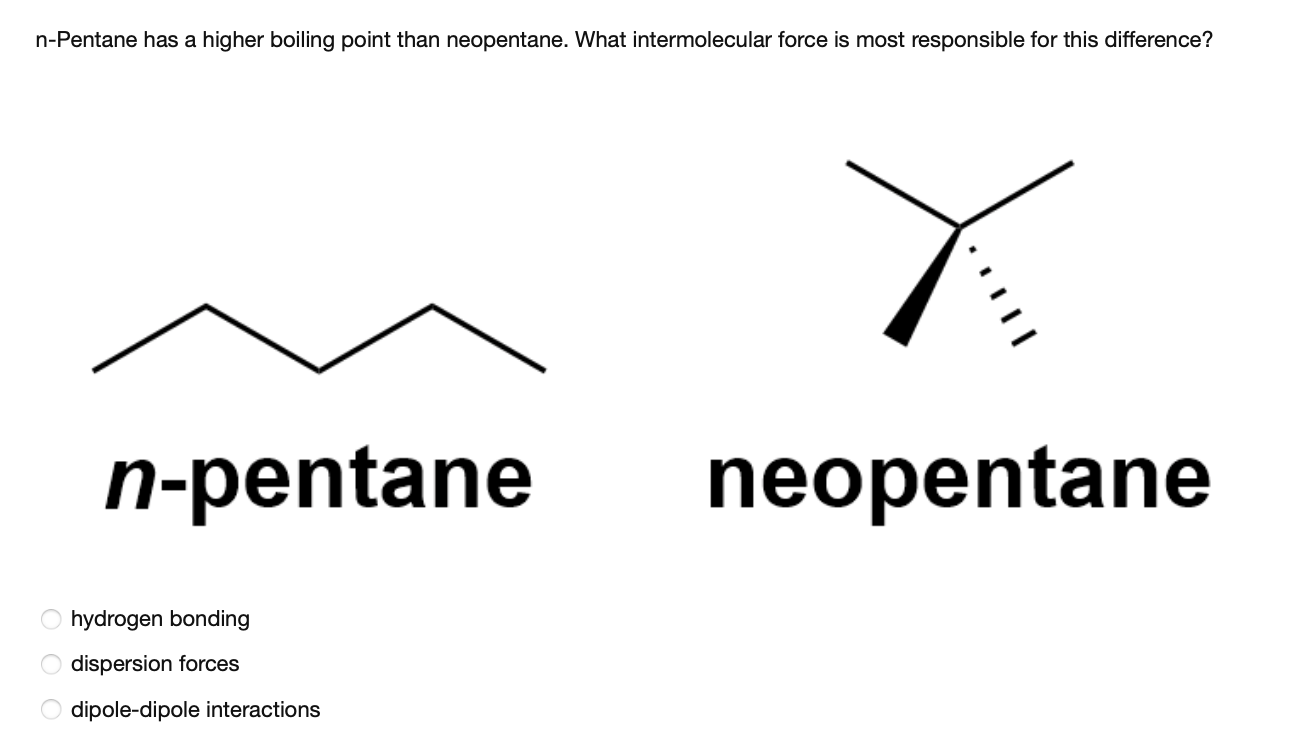
Statement-1: n-pentane has higher boiling point than neopentane and Statement-2: Larger surface area is responsible for greater van der Waal's force of attraction.
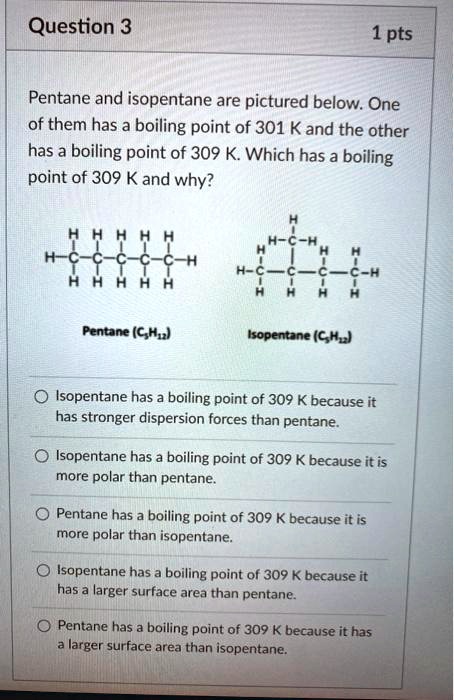
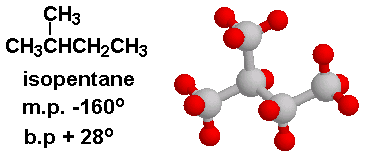
SOLVED: Question 3 1 pts Pentane and isopentane are pictured below. One of them has a boiling point of 301 K and the other has a boiling point of 309 K Which

Melting And Boiling Point Npentane Nhexane Stock Illustration - Download Image Now - Acid, Alcohol - Drink, Atom - iStock

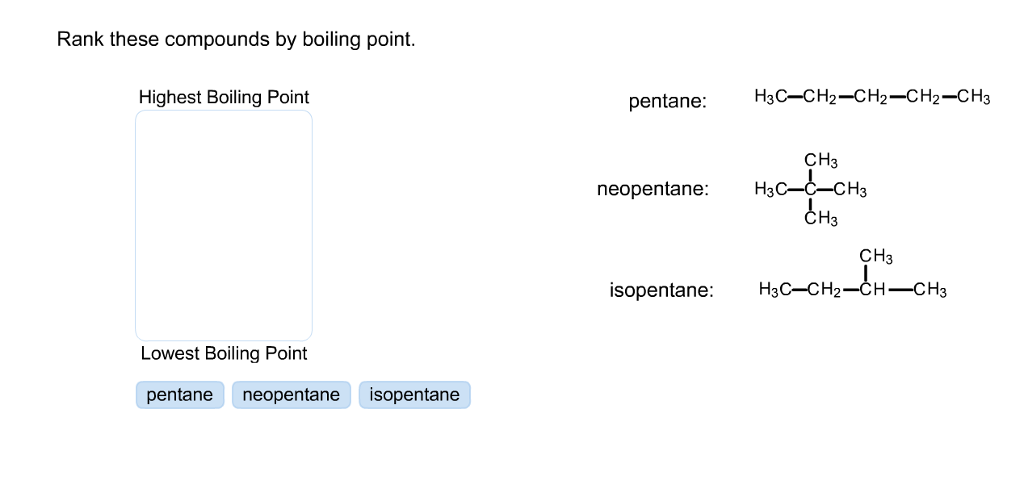
Rank these compounds from highest to lowest boiling point. a. pentane b. neopentane c. isopentane | Homework.Study.com